General information
Ovarian cancer often has no symptoms in the early stages. Later stages are associated with symptoms, but they can be non-specific, such as loss of appetite and weight loss. Cytoreductive surgery and HIPEC is a treatment option for treating ovarian cancer.
There are 4 main scenarios where surgery might be considered for ovarian cancer
1. Interval surgery
This is where women with newly diagnosed ovarian cancer undergo surgery after 3 cycles of chemotherapy and then have another 3 cycles of chemotherapy afterwards.
2. Upfront surgery
This is where women with newly diagnosed ovarian cancer undergo surgery first and then have 6 cycles of chemotherapy.
3. Surgery for relapsed ovarian cancer
This can also be done before or after further chemotherapy.
4. Surgery of bowel obstruction
Due to recurrent ovarian cancer.
At the time a new diagnosis of ovarian cancer is made the gynaecological oncologist will make a decision as to whether surgery should be the initial, or ‘upfront’, treatment or whether there would be a benefit to have chemotherapy first and then have ‘interval’ surgery. Interval surgery is usually recommended when the ovarian cancer is advanced and the surgeon is concerned that complete removal of all of the cancer may not be achieved at the time of upfront surgery.
Clinical trials
A clinical trial has been conducted in which women undertook 3 cycles of chemotherapy with carboplatin and paclitaxel chemotherapy. This was followed by surgery and then a further 3 cycles of carboplatin and paclitaxel. Half the women received HIPEC with cisplatin during their surgery.
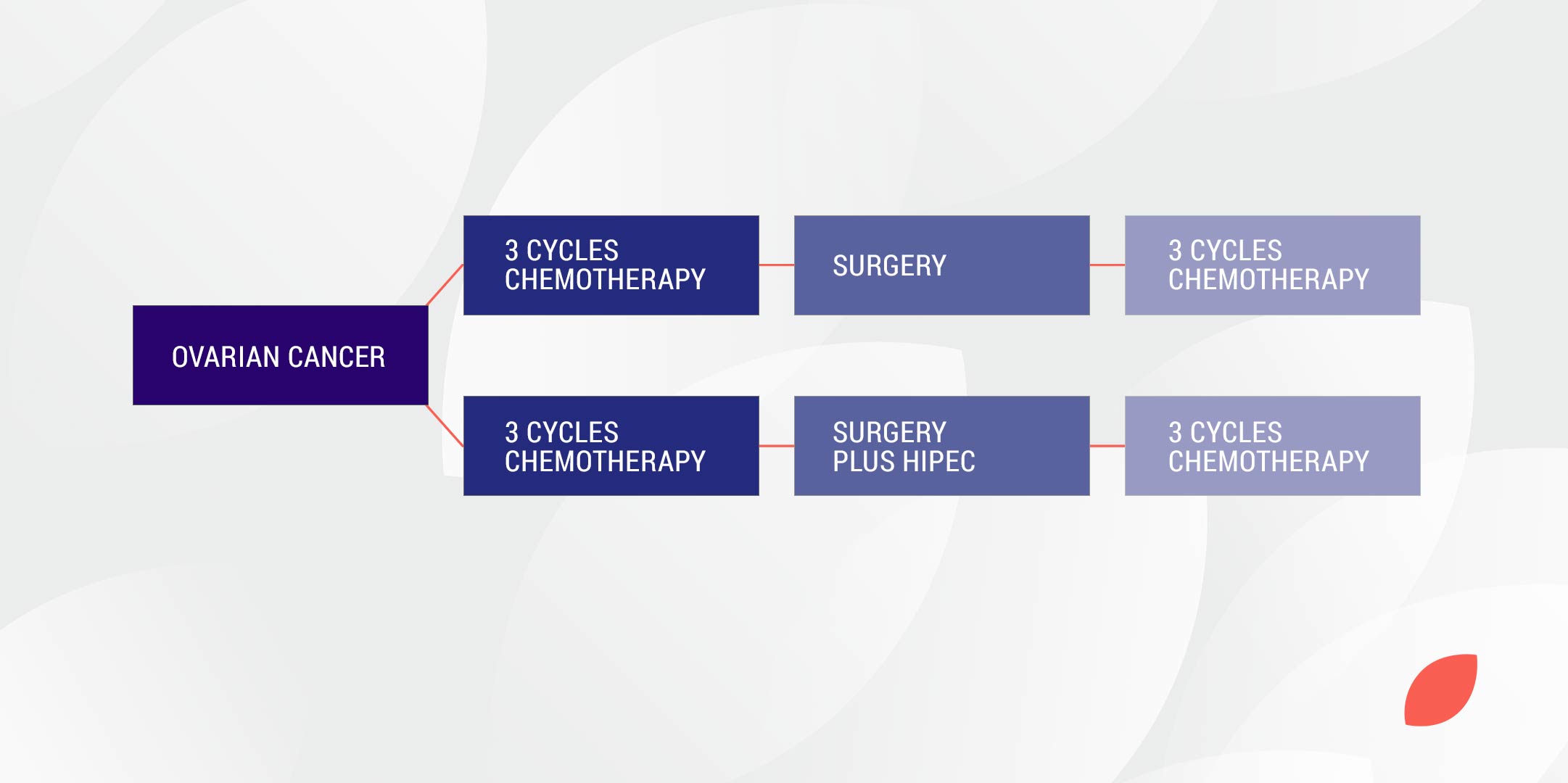
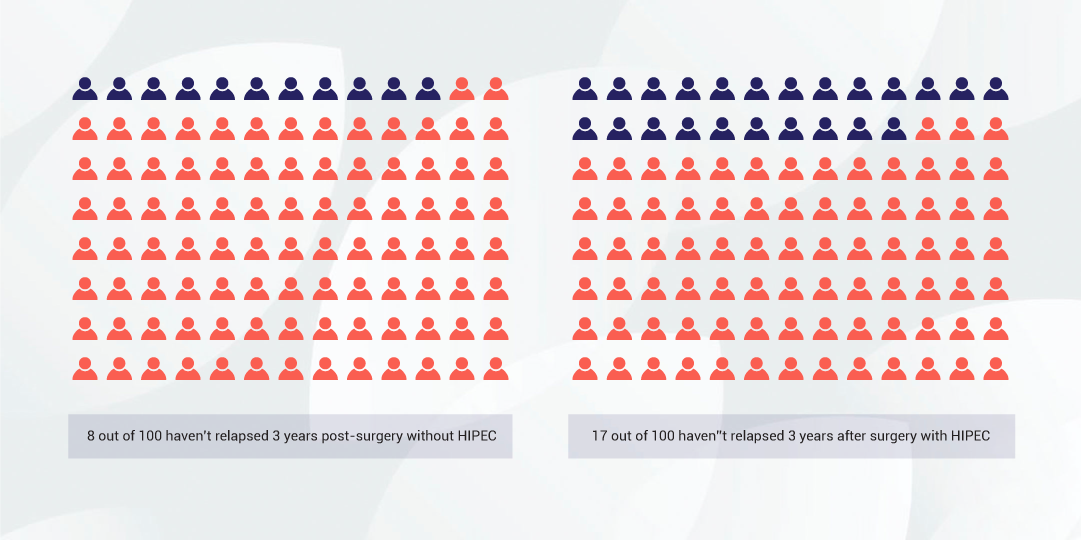
The results of this trial were as follows
The time it took for half of the women to relapse after treatment increased from 10.7 months with no HIPEC to 14.2 months with HIPEC. After 3 years 8 out of 100 women (8%) had not relapsed in the no HIPEC group versus 17 out of 100 (17%) in the HIPEC group. In this study relapse was sometimes detected based on blood tests and before recurrence was evident on scans or before the women had symptoms or relapse.
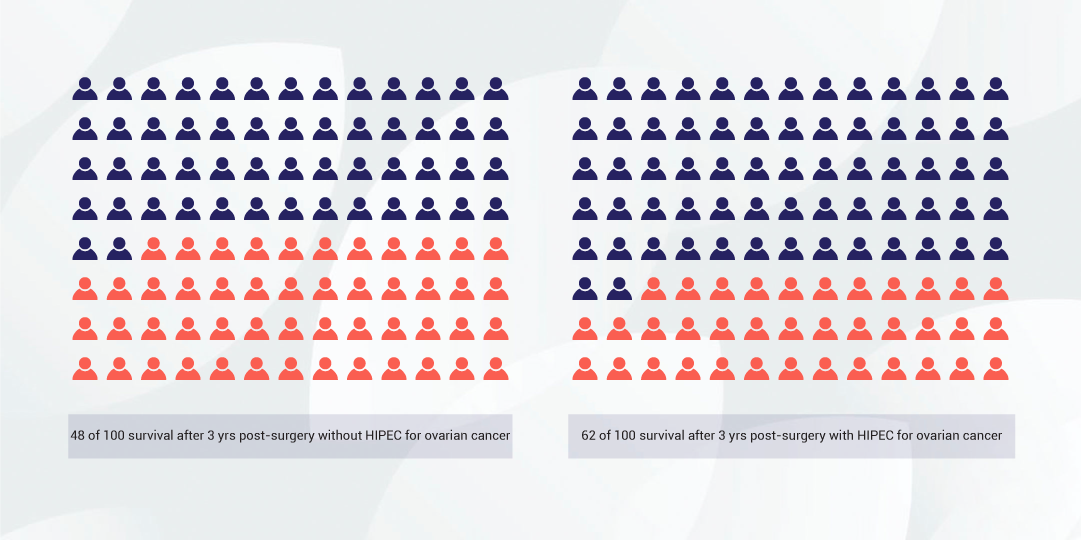
Median survival time refers to the timepoint after treatment at which half the participants have died and half are alive. The median survival time increased from 33.9 months in the no HIPEC group to 45.7 months in the HIPEC group. After 3 years 62 out of 100 women (62%) were alive in the HIPEC group compared to 48 out of 100 (48%) in the no HIPEC group.
In summary the addition of HIPEC translated into benefits in terms of time to relapse and overall survival.
A serious side effect requiring prolongation of stay in hospital or medication intervention occurred in 25 out of 100 women in the no HIPEC group and 27 out of 100 in the HIPEC group. The common side effects included abdominal pain, infection and ileus (slow bowel recovery that delays eating). A theoretical risk of the cisplatin chemotherapy is severe kidney damage but this was not observed in the trial as an antidote called sodium thiosulphate was used to protect kidneys.
In the trial the chance of death was less than 1 out of 100 (1%). The average time in hospital was 8 days for the no HIPEC group and 10 days for the HIPEC group. Time to recommencing chemotherapy was similar between the two groups (30 – 33 days) and over 90% of women completed treatment in both arms of the trial. Quality of life was similar in both arms of the trial.
Women who had HIPEC were more likely to need a stoma – 72 out of 100 (72%) in the HIPEC group versus 43 out of 100 i(43%) n the no HIPEC group. Stomas are created during surgery to avoid post-operative anastomosis (bowel join site) breakdown. Stomas are often reversible but may not be reversed in the event of relapse or sometimes because of the site that bowel was removed.
The results need to be understood in the context of the patients that participated. Women were selected for interval surgery after pre-operative chemotherapy as they were identified as having advanced disease with a lower chance of achieving complete surgical resection with upfront surgery. Approximately 1 out of 10 women (10%) of women having pre-operative chemotherapy will not progress to surgery due to lack of response to treatment. In this trial 2/3rds of women undergoing surgery were able to have all of their cancer resected.
Factors to balance in deciding to have chemotherapy, surgery and HIPEC compared to chemotherapy and surgery without HIPEC
- Greater chance of bowel resection and stoma/ostomy with HIPEC
- Similar chance of side effects
- Similar time course of treatment
- Similar completion rate for treatment
- Similar quality of life
- Longer time to relapse
- Longer overall survival
Currently HIPEC used at the time of upfront surgery is being studied in clinical trials. HIPEC has been studied in the setting of relapsed ovarian cancer and appears to provide a benefit. HIPEC is not routinely used in the setting of surgery of bowel obstruction from ovarian cancer but may be recommended at the discretion of the surgical team if there is ascites (abdominal fluid) present at the time of the surgery.